Foreword
Michelle Mitchell
CEO, Cancer Research UK
One in two of us born since 1960 will hear the words “you have cancer”. None of us live a life unaffected by cancer in some way. It is a disease of extraordinary complexity, but through research, we are slowly unravelling its complexity and unlocking better ways to prevent, diagnose and treat it.
Thanks to research, more people now survive cancer than die from it. This is the result of immense effort and collaboration and is progress we should all be proud of. But we cannot be complacent. Cancer outcomes in the UK lag behind comparable countries and there are still profound inequalities across the cancer pathway. We are simply not satisfied with the rate of progress we see in cancer – and we must demand better.
We feel particular urgency following the COVID-19 pandemic, which has had a devastating impact on people affected by cancer. Thousands of people have faced delays to screening appointments, investigations, treatment and clinical trials. We fear that the pandemic will have a long tail of impact on cancer – in terms of the direct effect on people diagnosed with cancer, by worsening inequalities, and in the longer term through the financial impact on funders of cancer research.
This timely publication offers several solutions, on both the interventions that will improve outcomes in the immediate term, as well as the research that will drive long-term progress.
On Cancer covers several themes that will be central to our future progress against cancer – including cancer prevention, early detection and equitable access to advanced treatments and technologies. This collection also presents tangible recommendations about how the outlook for people affected by cancer could be improved.
But it also presents a hopeful vision for our future, a world where the burden of cancer is greatly reduced. Research is the key to improving outcomes in the longterm, and there is great scientific promise ahead. New techniques, technology, and data are revolutionising what’s possible, and will allow us to make faster progress in the decades ahead than the decades past. This future is not guaranteed – it will take focus, collaboration and funding. But it is possible, and it is one that we must all work towards.
If we realise this vision, we would make an incredible difference to the lives of the millions of people affected by cancer each year. We would also bring immense benefit to the UK economically, fulfilling the UK’s potential as a leading destination for world-class science.
It is my great pleasure to introduce On Cancer, which is yet another chapter in Policy@Manchester, The University of Manchester and the Manchester Cancer Research Centre’s contribution to this critical area. It is my hope that this publication will galvanise interest and debate amongst policymakers and the broader cancer community – people affected by cancer, researchers, and everyone who shares a desire to make progress against this disease.
Disadvantage and disease: Finding solutions to inequalities in cancer
Dr Philip Crosbie, Dr Suzanne Johnson, Professor David Shackley

The COVID-19 pandemic has brought renewed focus to the great health inequalities between different communities in our society. Looking at cancer care, these inequalities exist across the whole cancer pathway from uptake in screening, likelihood to present early with symptoms, participation in clinical trials, diagnosis and access to treatments. Cancer is one of the leading causes of death globally with 1 in 2 people in the UK being diagnosed in their lifetime, and 28% of people in Greater Manchester dying from the disease each year. In the UK alone, there are around 20,000 extra cancer cases in the most deprived areas. So, what is being done to examine this issue, and what can we do to tackle it?
A combination of causes
Differences across the cancer continuum are born from a combination of structural inequalities, socioeconomic circumstances, socioenvironmental behaviours and lifestyle choices. Gender, racial and educational inequalities also play a part. These complex factors often influence generations and can result in deep-rooted fear and mistrust within communities, leading to barriers to accessing healthcare.
Predominantly, deprivation and lifestyle choices seem to be the most significant contributors, with Cancer Research UK estimating that lifestyle choices cause 40% of our cancers. Greater Manchester is significantly affected by health inequalities. It is the 38th most deprived Sustainability and Transformation Partnership (STP) area in England (of 42), and as a measure of lifestyle choices, it has the 39th highest smoking rate, which is 2% above the England average. These factors go some way to explaining the higher cancer incidences and mortality rates experienced in Greater Manchester compared to other, more affluent areas of the country.
People from the most disadvantaged socioeconomic backgrounds are less likely to attend cancer screening tests and thus likely to present later with their disease. We know that late stage at presentation is a bad prognostic marker: national data show that 49% of the most deprived quintile have early stage at diagnosis versus 58% for the least deprived. The stage can have a huge impact: for example in colorectal cancer, of the four stages, stage one patients have a greater than 90% chance of surviving five years after diagnosis as opposed to stage four patients where the figure is less than 10%.
Additionally, the under-representation of these groups within clinical trials limits the evidence available for better understanding their disease. This means there is a relative lack of understanding of cancer drivers in many marginalised groups, and this limits our ability to tackle it.
In its starkest terms, those who are most disadvantaged in life to begin with, are also most likely to be disproportionately and unfairly affected by a cancer diagnosis, detection and treatment.
From tradition to inclusion
Such disparities need addressing in ways which move from standard, traditional care packages into more inclusive, personalised and diverse packages that benefit those most in need. The pandemic, in revealing the extent of these disparities in healthcare, has proven that it is now essential that they are addressed to achieve significant and lasting change. The health disparities across the cancer continuum can be addressed by interventions such as community screening, community engagement and home-based testing.
Pioneering the move from healthcare models that deliver traditional care and detection packages to those that are more inclusive, diverse and community-focused are essential in helping to redress these deep-rooted health inequalities.
The Lung Health Check pilot
Lung cancer is a deadly disease with a five-year survival rate of only 16% after diagnosis. As lung cancer is most prevalent in areas of deprivation and is a driver of health inequality, it’s a priority for action. In short, lung cancer is by far the biggest cause of cancer death, and those deaths are concentrated in deprived communities.
The ‘Lung Health Check’ (LHC) was an initiative trialled by the lung cancer team at Wythenshawe Hospital (Manchester University NHS Foundation Trust), in partnership with Macmillan Cancer Support. The project took mobile screening units and placed them in public spaces across Manchester that were easy to spot and access for anyone without transport. They advertised their activity as a ‘lung health check’ to dispel fears around cancer and the perceived ‘death sentence’ of a potential lung cancer diagnosis. The locations offered an opportunity to engage with health professionals in a much more informal setting compared to a traditional clinic.
The benefits of the LHC approach were soon clear. It delivered a high number of early detection diagnoses (79% diagnosed at stage one vs a national average of 19.5%) and it engaged with those communities most at risk of this particular cancer. The project showed it could improve patient outcomes and reduce the burden of advanced disease and palliative care costs for the NHS.
The pilot showed a threefold increase in detection of lung cancer at its earlier stages where the tumour is 90% curable and the project has now been rolled out on a local and national scale through the National Targeted Lung Health Check programme, funded by NHS England.
A holistic approach
By taking a holistic approach, other diseases can also be detected at the same time such as Chronic Obstructive Pulmonary Disease (COPD) which is far more common than lung cancer with higher death rates, along with cardiovascular disease. Using the opportunity presented by the Lung Health Check initiative to screen for these three leading causes of premature death will engage hard-to-reach communities and reduce costs to the NHS and patients of late diagnosis. Increased participation of individuals from disadvantaged groups will also have the benefit of increasing their representation within clinical studies and help inform future treatments.
To fully engage with communities, it is vital to reach out to them, make connections and build trust to bring them closer to primary care providers and researchers. It is also essential to capture lived experiences. Multiple initiatives have demonstrated this approach successfully, leading to various forums, patient and public engagement groups and long-term collaborative partnerships. The objective is to build long-lasting and sustainable relationships with communities that levels-up healthcare provision and basic research. By achieving this, individuals will feel their voice can be heard and some of the long-standing issues facing certain communities can be tackled.
Professor Emma Crosbie, Division of Cancer Sciences at Manchester is also working to develop techniques of at-home screening that can help to address cancer inequalities, specifically in female cancers. Lower screening rates found within marginalised groups are posing a problem in gynaecological cancers. An example is work to develop at-home urine tests for Human Papillomavirus (HPV), a precursor for cervical cancer, which it is hoped could address barriers to screening and contribute to improved health equity.
Reaching all communities
Engagement with those communities most at risk of health inequalities such as late cancer diagnosis is vital if a shift to early detection and improved patient outcomes in all communities is to be realised.
Leaders and policymakers within our healthcare service need to put urgent thought into how our system can be structured towards earlier interventions. Increased and meaningful community engagement is key to achieving equitable healthcare and has the added benefits of reduced strain on the NHS from the impacts of late diagnosis. A robust and well-resourced infrastructure is needed to support this.
Barriers and the opportunity for change
There are numerous challenges that come with moving to a diverse healthcare package that engages with disadvantaged and marginalised communities. Implementing successful large-scale community screening involves overcoming problems with the logistics of being based in public spaces, capacity issues, technology barriers and workforce limitations.
Looking at imaging as a diagnostic test for cancer, the UK currently has fewer radiologists per head of population compared with the European average. 2015 figures showed an estimated 4.8 consultant radiologists per 100,000 people. This is one of the lowest figures in Europe, comparing to a mean of 12 radiologists per 100,000 population for Western Europe. These limited numbers of Thoracic Radiology Specialists means reporting on such targeted lung cancer screening scans is problematic in the long term. Similarly, the UK has a significant shortage of CT scanners versus other Organisation for Economic Co-operation and Development (OECD) countries, with latest 2020 To fully engage with communities, it is vital to reach out to them, make connections and build trust to bring them closer to primary care providers and researchers. Leaders and policymakers within our healthcare service need to put urgent thought into how our system can be structured towards earlier interventions. 8 9 data showing the UK has 9.0 CT scanners per million population versus 35 in Germany, 42 in the US, and 70 in Australia.
Restructuring our healthcare system towards prevention and an early detection approach requires reversing the diagnostic and treatment pathway as well as additional funding for services that support early detection. This will also require policy-makers to tackle perceived cultural barriers to encourage screening uptake and engagement with primary care providers.
The Department for Health and Social Care and NHS England have the opportunity to develop innovative cancer services that can reach and engage with all communities. A move towards inclusion is essential not only for those communities who are currently suffering from poor healthcare and the effects of late diagnosis, but also for our healthcare system which isn’t sufficiently resourced to take on the increasing burden of palliative care and treatments which result from these inequalities.
We now need robust and clear leadership on the development of a services, infrastructure and workforce development plan that will support this already world leading detection methodology.
The solutions lie in supporting communities to make healthier choices, making access to healthcare convenient, and reducing unwarranted variation in healthcare through tactical interventions.
Dr Philip Crosbie is a Senior Lecturer in the Division of Infection, Immunity and Respiratory Medicine at The University of Manchester and an Honorary Consultant in Respiratory Medicine based at Wythenshawe Hospital, Manchester University NHS Foundation Trust. His research and clinical focus is the early detection of lung cancer
Dr Suzanne Johnson is a lecturer in the Division of Cancer Sciences and Division lead for Social Responsibility with particular interests in developing inclusive practice in research and teaching and addressing health inequities. Her background is in basic and translational cancer research, and in 2020 Suzanne was appointed Programme Director to develop a new online Masters programme in Transformative Oncology with The University of Manchester Worldwide.
Professor David Shackley is the Clinical Lead at MAHSC (Manchester Academic Health Science Centre) for cancer working with colleagues to bring research and clinical care closer together. He is the Director of the Greater Manchester Cancer Alliance, and a Consultant Urological Surgeon. From 2015 to 2018 he was the Medical co-lead for the National Cancer Vanguard which brought GM and London together as a 10 million catchment population to develop and test new cancer approaches before a broader roll out across NHS England.



Access and inclusion: Can we move cancer services closer to home?
Dr Philip Crosbie and Dr Dónal Landers

Traditionally, the vast majority of cancer services take place in hospitals, but new thinking and new technology are rapidly changing this landscape, particularly because of the impact of COVID-19 on the delivery of healthcare services. Delivering cancer services out of the hospital in the community or in the home can offer benefits for both the patient and the healthcare professional, as well as offering potential savings in expenditure. But more should be done to ensure the right protections and incentives are in place, and that barriers are removed to enable this new and promising reconfiguration of cancer care delivery.
A changing landscape
Using new technologies and new methods, it is now possible to move some of the components of cancer care away from the hospital and closer to the home. One way of achieving this is through the monitoring of patients using wearable devices. This enables a structured and continual collection of data from the patient at home, allowing the doctor to assess their progress in a more rigorous way and make decisions accordingly.
In addition, interventions such as community screening help with early detection and therefore improved patient outcomes and can more easily reach socially and economically disadvantaged communities where take up is generally poor. This improved prevention and early detection lessens the strain on the healthcare system by reducing the numbers of patients with advanced disease.
This is not to say that all cancer services ought to be brought closer to the home, and barriers such as the ‘digital divide’ (inequalities of access to internet and other technologies) must not be overlooked. Identifying services that could be moved from the hospital into communities will provide better patient outcomes for our population, as well as setting positive models for healthcare systems around the globe.
Research innovation and design
The University of Manchester’s digital Experimental Cancer Medicine Team (digital ECMT) aims to empower patients and healthcare professionals to innovate and design new cancer care pathways. The digital ECMT has developed a proof-of-concept study, IN-HOME, which assesses the feasibility and clinical benefit of in-home sampling, using a device and smart phone application for detecting Acute Kidney Injury (AKI) in patients receiving cancer treatments.
The aim of this study is to understand whether self-monitoring of creatinine levels using a drop of blood and a creatinine measurement device (provided by Nova Biomedical) would lead to early detection of AKI. This study is progressing to its second stage (clinical benefit), and if successful could improve both patient quality of life and patient outcomes. Importantly, it may enable patients with chronic kidney disease, but with stable renal function to enrol in future clinical trials, where most are currently deemed clinically ineligible.
Another trial, NOTION (in-home sampling of cytokines in immunotherapy patients) is a proof-of-concept study run by the digital ECMT which will investigate the collection and measurement of ‘dry blood spot’ samples at home. The primary objective of this trial is to evaluate the feasibility of collecting and measuring cytokines in the blood of patients receiving immunotherapies. The aim is to develop in-home analysis that can predict immune-related toxicities giving clinicians the information they need to avoid damaging inflammatory side effects.
By increasing frequency and accessibility of toxicity monitoring, both of these interventions may impact on care pathways to allow for earlier intervention, reduce treatment complications and potentially increase the time a patient can stay on treatment.
Community screening and engagement
Community screening is a convenient alternative to hospital screening for many communities, particularly those in isolated locations and in areas of deprivation.
In partnership with Macmillan, the lung cancer team at Wythenshawe Hospital (Manchester University NHS Foundation Trust) established the Lung Health Check (LHC), a community-based lung cancer screening service held in convenient community locations. This LHC programme addressed perceived barriers to screening through improving convenience and accessibility while also promoting the message of early detection and a ‘curable’ stage of cancer. Most attendees were from disadvantaged areas, which evidenced the success of this health check in breaking down perceived barriers. 80% of detected cancers were early stage and therefore met the goal of early detection and prevention.
This pilot project has been scaled up locally and nationally through the National Targeted Lung Health Check programme funded by NHS England. It shows the importance of screening to achieve early intervention and reduce lung cancer mortality. It is hoped that a national lung cancer screening programme can be established based on the evidence from the LHC approach.
Cancer services beyond hospitals – future needs
Our current healthcare system is predominantly designed around detecting cancers at the latter stages of their progression. A shift to prevention and early detection is required to improve patient outcomes. Moving some screening and monitoring to the community or home is necessary in order to achieve this.
With community screening, it is vital to focus on communities where screening take-up is limited. This dynamic switch in attitude requires a measured approach and it is important that steps are taken to mitigate any socio-economic divides being translated into digital divides. It is essential, therefore, that screening is tested in and for the real world, external to clinical trials.
Other barriers to implementing community screening include the logistical challenges of integrating Wi-Fi in mobile units, the current NHS capacity of radiologists and software challenges of combining patient records while complying with current GDPR regulations. Additionally, we need to know that technologies are assessed to ensure we understand any risks and are clear on the benefits. We also need to understand how they change the way health professionals engage with patients and be aware of any negative repercussions.
These critical areas require leadership from the Medical and Healthcare products Regulatory Agency (MHRA) and National Institute for Health and Care Excellence (NICE). Both regulators have a role to play in evaluating and approving the technologies and care pathways necessary to move cancer services out of the hospital and into the community.
At a national policy level, critical attention must be paid by NHS England and the Department for Health and Social Care, with all regulators and policy-makers working in collaboration with experts, using evidence from comprehensive studies.
We cannot afford to ignore the great benefits of transitioning further cancer care from hospitals into our communities and homes. But policy decisions around implementation of new ideas and technology in an ethical way must be grounded in rigorous research with the needs of all patients, particularly the most disadvantaged, at their heart.
Dr Philip Crosbie is a Senior Lecturer in the Division of Infection, Immunity and Respiratory Medicine at The University of Manchester and an Honorary Consultant in Respiratory Medicine based at Wythenshawe Hospital, Manchester University NHS Foundation Trust. His research and clinical focus is the early detection of lung cancer.
Dr Dónal Landers is Director of the digital Experimental Cancer Medicine Team, Cancer Research UK Manchester Institute. He is a Clinician and a Fellow of the Faculty of Pharmaceutical Medicine with over 25 years of experience and achievement in early clinical development, clinical practice, healthcare and pharma consulting, and delivering digital health innovation and solutions



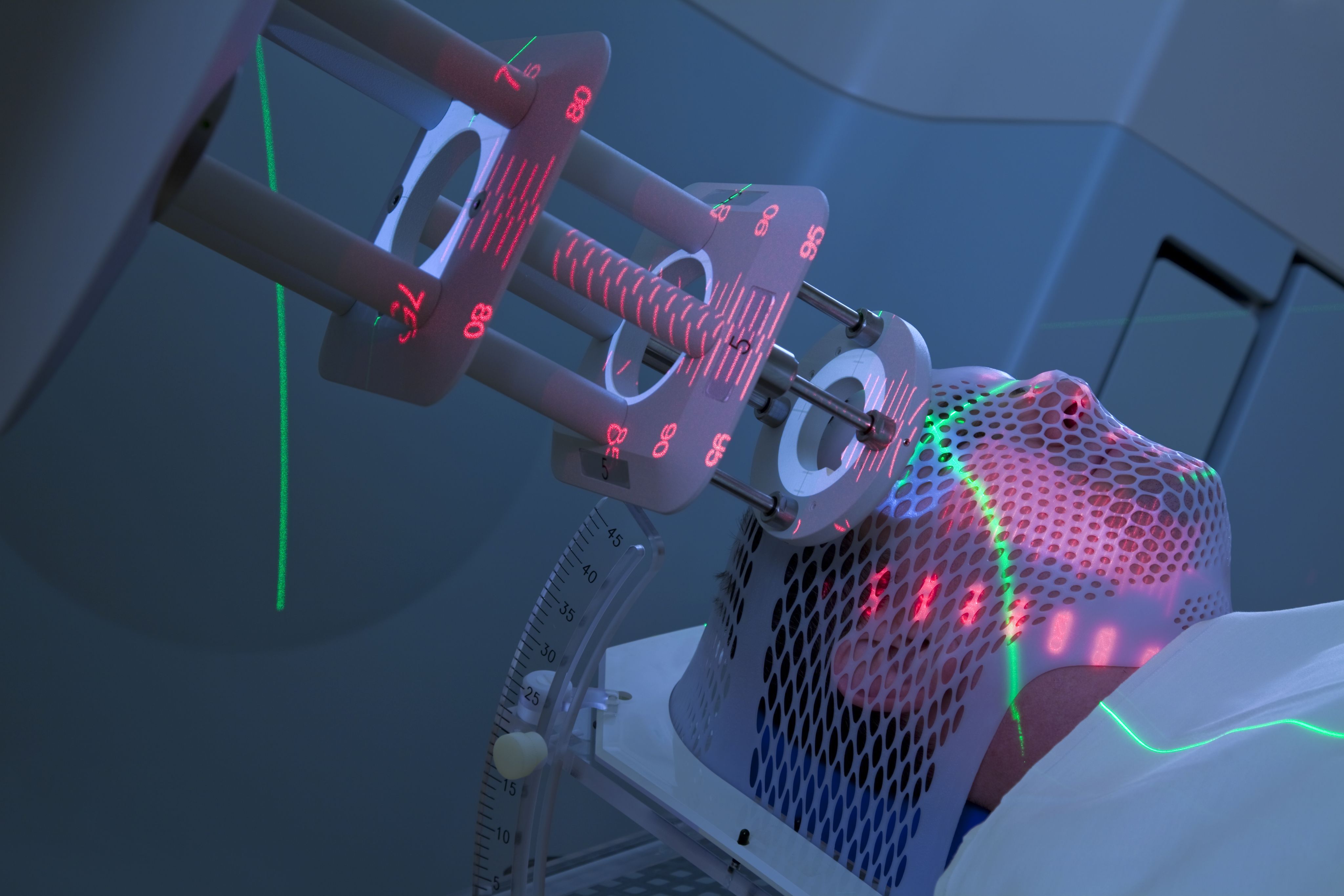
Advanced radiotherapies: What are the challenges and opportunities?
Professor Karen Kirkby and Professor Ananya Choudhury

Advanced radiotherapies are redefining the kinds of cancer treatment that are possible. These developments are exciting, but they also present new challenges. One challenge for researchers and clinicians is how to support policy-makers, tasked with developing treatment and care standards across the NHS. So, what are these new treatments, what do they mean for patient choice and what steps can we take to make the most of these opportunities?
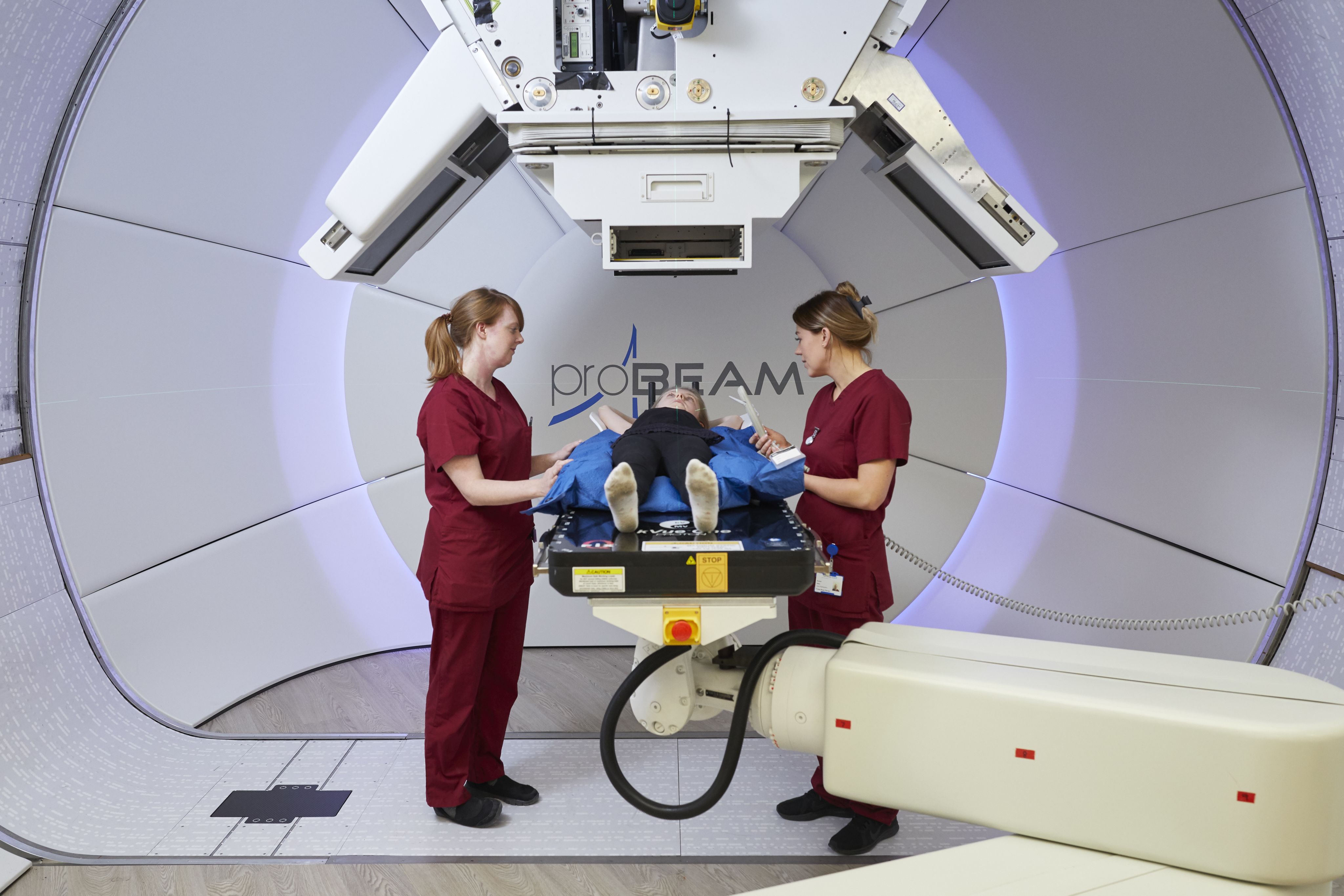
Proton Beam Therapy
The Proton Beam Therapy (PBT) Centre at The Christie in Manchester was the first NHS site in the UK to provide radiotherapy using high energy protons to the public. The Centre houses a state-of-the-art particle accelerator that accelerates protons to two-thirds of the speed of light, before delivering them to the tumour site. This precision means that protons can be used in very complex radiotherapy treatments for example treating tumours that are dangerously close to critical organs. Proton therapy is also used for treating children as it causes less damage to healthy tissues and so reduces the chances of ‘secondary malignancies’ (cancers which may have been caused by previous cancer treatments) later in life.
There is a national ‘Indications List’ of the types of tumours proton therapy can best be used on. Referrals are made by clinicians and approved by a national panel with discretion to approve treatments for non-listed tumours, if the tumour location or patient’s anatomy mean that protons are likely to be the most effective method. This provides a valuable flexibility in determining the best course of action for every patient.
FLASH radiotherapy
Another new type of advanced radiotherapy, known as FLASH radiotherapy, is also showing promising signs of becoming a new precision treatment for cancers. At The Christie researchers are using FLASH proton beams in the research room in the clinical PBT centre. FLASH uses very high dose rates and appears to spare normal tissue while still destroying the tumour. Researchers, including teams at The Christie, are undertaking research to understand the mechanisms that cause the FLASH effect. This will also help to understand how FLASH might best be used in the clinic and which patients might benefit most.
MR-LINAC: Integrating imaging directly into treatment
Here at The Christie in Manchester, we have also been developing the use of a new radiotherapy technology known as MR-LINAC. This technology combines Magnetic Resonance (MR) imaging with a photon Linear Accelerator (LINAC), and performs MRI scans during treatment. We are now investigating the potential of this innovation to help clinicians tailor treatment choices based upon an enhanced understanding of the individual patient’s response. MR-LINAC technology offers us the potential for realtime monitoring of the tumour and normal organs during a treatment course, and the agility to adapt that treatment based on what we see. This integration of sophisticated imaging technology directly into the radiotherapy treatment pathway is another breakthrough for Manchester’s cancer sciences research that will enable a new level of personalised, precision treatment.
MR-LINAC treatment is more expensive than standard treatment as it takes longer to deliver and requires more expert input from healthcare professionals (a one hour appointment slot versus a twenty minute appointment), so the gains must be balanced by the increased cost in resources.
These techniques are so early in their development that we are still conducting the research that will allow us to decide the best ways in which this technology can be used in the health service. This evidence will also allow researchers and policy-makers to produce a full costbenefit analysis of material costs, changed outcomes, and overall viability of MR-LINAC treatment as part of the treatment standard for different forms of cancer.
New advances and patient consent and choice
Another new development that we are working on relates to informed consent and the involvement of patients in determining their treatment. Advances in clinical research and technology have allowed us to start work on forms of ‘patient-guided’ radiotherapy, particularly in cases of head and neck cancers. These techniques allow clinicians to present patients with a toolkit that will allow them to understand and play an active role in choosing how their treatments are designed. For example, we will be able to ask patients whether they would rather choose a course of therapy that may impair their hearing against another which might impact their ability to comfortably swallow. Of course, choices like these will not be happy ones, but by bringing the patient in as a fuller participant in their own treatment, we get their full and informed consent for their treatment course. This approach also offers the patient the agency and dignity involved in taking some control over the direction of their treatment.
Ideas and implications
In the field of FLASH radiotherapy, the Cancer Research UK ‘Radiation Research Network’ (RadNet) is currently bringing together clinicians, clinical scientists, radiographers and researchers to discuss which tumours might benefit most from FLASH and start develop of a potential ‘indications list’ for FLASH therapies. The development of this at a national scale (modelled after the existing arrangements for PBT), will be the next step if FLASH radiotherapy delivers on its promises. We see a role for policy-makers in the Department for Health and Social Care, and for parliamentarians with interests in cancer and radiotherapy, to help accelerate the process of getting from clinical discussion into policy and practice.
As researchers, we also seek engagement with and leadership from health policy-makers on the new opportunities for enhanced and informed patient consent. For example, should the use of toolkits such as the one we are developing for head and neck treatments become the norm for bringing patients into decision-making around their own treatments? We are beginning to see how technologies such as the MR-LINAC can play a role in supporting patient choice. A better understanding of treatment response means greater capacity to advise and empower patients to help determine their course of treatment.
While these technologies continue to develop, the discussion about their potential use should be led by the Department for Health and Social Care. It should also involve the National Institute for Health and Care Excellence (NICE), NHS England, NHSX, and the Medicines and Healthcare products Regulatory Agency (MHRA), as well as clinical experts from across the world. This dialogue will benefit from integration into policy discussions via a work programme that we can help to develop between these agencies and the clinical research community.
Building connections, creating new standards
Building and maintaining the connection between advanced radiotherapy research and policy-makers is essential and often overlooked. We as researchers need to show what PBT can achieve and how it can best fit into the treatment landscape. However, we need to understand more about how non-clinical constraints affect public policy and have a regular and ongoing correspondence with decisionmakers to give them a better idea of where our research can help achieve their objectives and, importantly, where it can’t.
In making these recommendations – for supporting the development of a FLASH indications list and national panel formation; for developing a formal work programme to discuss new standards for informed consent in treatment pathways; and for maintaining a sustainable and dynamic forum for correspondence between researchers and policymakers – we seek to show policy audiences the next steps that we need to take.
These clear, measurable, and reachable goals will have far-reaching benefits, moving us closer to the cancer health outcomes made possible by these advanced new technologies.
Karen Kirkby is Professor of Proton Therapy Physics - a joint post between The University of Manchester and The Christie. Karen is responsible for developing a programme of international leading proton research and innovation to deliver direct patient benefits. This goes from basic research, through pre-clinical and translational research to clinical trials.
Professor Ananya Choudhury is Chair and Honorary Consultant in Clinical Oncology at The Christie and The University of Manchester. Ananya specialises in urology and has a strong interest in translational research. She is clinical lead for advanced radiotherapy including the MR-LINAC project and is co-Group Leader of The Translational Radiobiology Group within the Division of Cancer Sciences.
Rules of the road: The need for new quality standards for AI technology in healthcare
Dr Dónal Landers and Dr Gareth Price
In recent years, we have seen a profusion of AI, algorithm, and machine-learning technologies enter clinical practice. This has happened across the health and care sector, and cancer testing and treatments have been no exception, with some proven benefits. For example, AI has been shown to be capable of recognising patterns in scan images that the human eye would have difficulty detecting. These developments could open a new horizon for earlier diagnoses, as well as informing treatment choices. However, there are risks as well as rewards, which begs the question: are the policies and regulations we need in place?
Data matters
One core concern with the development of algorithms in clinical practice is the quality of the datasets upon which they are built. If an algorithm has old datasets, or low quality and low fidelity data at its foundation (in other words, the information is potentially out of date or inaccurate), an algorithm (or any other use of AI in the healthcare context) cannot consistently reach the best decisions for patients. If such an algorithm were implemented into clinical practice, the technology may even do more harm than good, and the confidence and trust of patients and practitioners will be lost.
Despite the huge numbers of algorithms developed by researchers and commercial companies, there are relatively few finding their way into clinical use. In part this may be due to a lack of trust in the way algorithms are trained (for example, the underlying data quality) and validated (for example, if compared against existing standard practices of care). Furthermore, it’s important to know why an algorithm is making a particular recommendation, and for the algorithm to contain knowledge that is not already well-known to clinical teams. These are the challenges facing algorithm developers, as they must prove the usefulness of their technologies before they are likely to enter clinical practice.
New research, greater transparency
The University of Manchester’s digital Experimental Cancer Medicine Team (digital ECMT) connects patients, clinical teams, technology and science. The team brings researchers, clinicians, patients and technology together to innovate in early clinical trials. Our aim is for patients, carers and families to work in partnership with researchers on clinical trials and new technologies.
Our team at the digital ECMT, as part of a collaboration with The Christie, developed an ethically designed algorithm as part of the CORONET.AI Decision Support System (https:// coronet.manchester.ac.uk). This is an online tool to support decisions regarding hospital admissions or discharge in cancer patients presenting with symptoms of COVID-19 and the likely severity of illness. The tool, utilises real-world patient data relating to the admissions and discharge of cancer patients presenting with symptoms of COVID-19. In addition to satisfying the General Data Protection Regulation requirements relating to the transparency of decision-making, our algorithm also meets the wider ethical needs for clearly interpretable and explainable results.
As a collaborative team, we have worked hard to ensure that the algorithm is ‘transparent’, so that the clinician can clearly interpret the results and, in turn, that these can be explained to the patient in ways they can understand. The ethical imperatives that underpin this are based on the key medical ethical principles of autonomy, beneficence, non-maleficence and justice.
Clearly, no ‘black box’ algorithm (one that cannot show the reasons behind its decision based on the data) can be acceptable for making clinical decisions. Firstly, clinicians will quite rightly refuse to work with these algorithm types. Secondly, patients will be leaving vital decisions about their health and treatments to a process that they cannot understand and therefore trust. However, these considerations also need to be seen in the context of the rapid proliferation of algorithms in both the formal and informal health spaces.
It is for these reasons that leaders in our health and care system need to put urgent thought into the standards that we, as a society, should demand from any deployment of artificial intelligence into all clinical decision-making processes.
The need for new ‘rules of the road’
Crucial to the successful deployment of AI and algorithms into our health and care system is the development of minimum acceptable standards for their construction and use. This is essential both to ensure the quality of the task that they carry out and to maintain the confidence of the clinician and the patient.
These standards must include:
- A robust framework setting standards for the age, quality and accuracy of the underlying datasets
- Clear standards for the transparency of the operation or the use of algorithms in clinical decision-making – decisions that are clear, interpretable, and explainable
- An agreed pathway for auditing and contesting decisions where AI has been used to determine a course of action We expect a rapid proliferation of AI tools over the coming years, making it vital that regulators and health system leaders act now to establish the ‘rules of the road’ for new entrants to this market.
Three national regulators have a key part to play:
- The Medical and Healthcare products Regulatory Agency (MHRA) are responsible for ensuring the safety of medical devices, which includes the use of software such as algorithms
- The National Institute for Health and Care Excellence (NICE) have responsibility for recommending the ways that tools (medicines, diagnostic tests, treatments, etc.) are used in healthcare
- The Care Quality Commission (CQC) has an extensive role in monitoring and evaluating the deployment of tools and technologies in healthcare settings.
All have a role to play in the development of a minimum standards framework for the entry of AI/algorithms into the lucrative clinical market. Working together, and with the advice of experts in both the health research and commercial sectors, they can ensure that only the safest and most effective products are made available to patients and clinicians.
Beyond the healthcare sector, there is evidence of this need. For example, a new industrial standard for AI is being developed by the British Standards Institute, and substantial work is going on in the European Union to enhance their own regulation of AI. Clinicians and researchers should partner with industry and regulatory bodies to keep quality and patient care at the heart of health innovation.
Huge promise
Ultimately, the use of AI and algorithms to improve healthcare holds huge promise. If done well, we will see better outcomes, quicker decisions, lives saved, and years added to lives, because of these new tools. In cancer, we can expect AI to continue to increase our ability to detect tumours earlier and to treat them more effectively.
With a responsible and ethical approach to the development and deployment of AI in healthcare settings, we can expect these new technologies to revolutionise cancer testing and treatments.
We now need robust and clear leadership from policy-makers and regulators to develop new national standards and build in the safeguards we need, to grow clinical confidence and public trust in these remarkable new tools.
Dr Dónal Landers is Director of the digital Experimental Cancer Medicine Team, Cancer Research UK Manchester Institute. He is a Clinician and a Fellow of the Faculty of Pharmaceutical Medicine, with over 25 years of experience and achievement in early clinical development, clinical practice, healthcare and pharma consulting, and delivering digital health innovation and solutions
Dr Gareth Price is a Senior Lecturer in the Division of Cancer Sciences at The University of Manchester and a clinical scientist at The Christie. His research focuses on the use of data collected during routine care to derive clinical insight, identify potential improvements in treatment, and provide evidence of the impact of innovations and changes to practice on patients’ clinical outcomes.


Data directly from our patients: Is improving patient data the key to better cancer care?
Professor Corinne Faivre-Finn, Professor Niels Peek, and Dr James Price

To provide the best care and support for cancer patients during and after treatment, it is essential to collect and work with data that captures patient experiences and patient-reported outcomes. But data is not a simple subject. The way healthcare services work with data, and how we work with patients to collect it, must be tackled from several angles. So, what can be done and what are the priorities?
Challenges with real-world data
To obtain real-world evidence, for example to establish the outcomes of a particular course of treatment and the lessons we can learn for future treatments, it is essential that researchers can easily access high quality real-world data collected (for example from patient electronic health records) and also patient generated health data. Data should be collected in a structured way both at the start of a patient’s journey (diagnosis) and during their treatment pathway. This will allow the capture of demographic, cancer-specific and survival data that could be comparable to the data collected through clinical trials, particularly if quality control processes are built in. At present, the quality of routinely collected data in healthcare settings is still substandard when compared to that gathered in clinical trials, where strict and rigorous processes provide quality assurance.
One of the challenges faced by researchers is the lack of data collected on the impact of a medical condition, such as cancer, and its treatment from the patient's perspective. Clinician-reported data has been found to frequently under-report the frequency and severity of symptoms experienced by patients with cancer. A possible solution is to collect patient-reported outcome measures (PROMs) which are self-completed questionnaires on symptoms and quality of life.
Another challenge is the governance associated with real-world health data. Data sharing is more complex since the introduction of the General Data Protection Regulation (GDPR). To overcome this barrier, an umbrella exemption (Section 251 approval), can be applied for, which first requires NHS ethical approval and a sponsor, and then further applications before permission is granted. Obtaining these umbrella approvals via Section 251 to undertake research with large, real-world datasets is challenging and maintaining public trust regarding data sharing and security is paramount.
Finally, while a positive data culture exists, it is currently hampered by a lack of time and training for most clinical teams who will be involved with the collection of the data.
ePROMS: New ways to capture and use data
The routine collection of electronic patient-reported outcome measures (ePROMS) helps to significantly improve the understanding of the side-effects from treatment and the quality of life for all patients.
The Christie Hospital in Manchester has set up ePROMS as a clinical tool to help improve the communication between patients and clinical teams. The aim is to better tailor treatments and aftercare as well as providing high-quality data to facilitate real-world research. This initiative, a collaboration between The Christie, The University of Manchester and Manchester Cancer Research Centre (MCRC), will also help to enhance many other aspects of routine patient care.
Despite the impact of COVID-19 the roll-out of ePROMS to date has been highly successful, and with funding from The Christie Charity, ePROMS will be rolled out to all relevant disease groups at The Christie by the end of 2022. In addition, a real-time responsive service for patients will be developed in response to patients’ reported symptoms. In this way, ePROMS will feed into models to predict clinical outcomes which may help clinicians to make decisions about treatments. ePROM data may also allow clinic appointments to be used more effectively for patients who require clinical input and reduce unnecessary visits for well patients who do not. Patient care pathways are already becoming personalised as a result of ePROM data. For example, patients receiving Herceptin and other anti-HER2 drugs following surgery for breast cancer are monitored with serial echocardiograms and ePROM questionnaires. Patients who report via ePROM that they are well with no symptoms are able to proceed with treatment without clinical review and patients reporting symptoms via ePROM receive clinical review.
Another recent development in improving the accessibility is our UK Computer Aided Theragnostics (ukCAT) project, launched at Manchester Cancer Research Centre and The Christie in 2017. This system makes anonymised routine patient data available to research organisations who can use those insights to develop new approaches to diagnoses and treatments.
Public consultation and how data is shared
Processes for sharing data in a way that are quick, transparent and acceptable to patients and the public is vital to the ambition to progress towards real-world evidence at scale. The University of Manchester has been working alongside national bodies, including the National Institute for Health Research (NIHR), NHSX, and NDG (National Data Guardian) to run public consultations on the advantages and risks of data sharing.
A recent public consultation, focused on health data sharing in the pandemic, used three citizens’ juries to weigh the benefits of valuable data systems against disadvantages of continuing to process data. Jury members were informed about what data sharing and electronic health records are, why they are important and what the data is used for. This way of bringing complex evidence to the public enabled juries to make judgements and reach reasoned answers to questions concerning data.
The consultation revealed that overall, juries supported decisions to introduce data initiatives during the pandemic and the majority were in favour of all data sharing initiatives continuing, as long as they were valuable. In addition, 94% of jurors believed that an independent body of experts should review data sharing initiatives, rather than those organisations responsible for the initiatives.
Additionally, MCRC’s Patient and Research Data Statement exemplifies the commitment to an ambition of using patient data in a safe and secure way to look at new innovative ways to improve patient outcomes. This has been accomplished through best practice related to patients in research programmes with strict security measures that protect patient confidentiality. Data collected includes real-world data combined with the Greater Manchester Care Record to improve individual cancer care, including diagnosis, treatment and monitoring of cancer. Our system uploads the symptoms, quality of life and experience of patients directly to central and secure data storage at The Christie. This can then be used by both clinicians and patients for them to jointly make informed clinical decisions.
Patient data and steps for the future
There are very few places that have been able to establish ePROMS, or similar patient data initiatives, within a routine setting. Such initiatives require high levels of expertise, engagement with patients and staff, validation of work and in-depth data analysis. To make them a successful and integral part of our own health and care system, we need to take a number of preliminary steps:
- Patient data must be properly linked between primary and secondary care. This results faster communication between both parties and ensures that all relevant information is available in both settings. Leadership from Department for Health and Social Care, NHS England and from our newly developing Integrated Care Systems is key to building this ability.
- To help develop a robust research culture, both current and future clinicians will need further training to understand the importance and clinical relevance of high quality data collection. Organisations like NHSX and Health Education England can play a leading role in setting these standards and delivering the necessary workforce and student training. This should involve a review of current provision of both data collection and quality assurance training, in addition to consultation with researchers and clinicians to develop new content appropriate to our rapidly advancing technologies.
- The process for obtaining permission for the use of data in clinical research must be made easier: opportunities are currently being lost because of the time and complexity required to access the data that researchers need to develop new techniques and treatments, and to co-ordinate healthcare more effectively between organisations within the NHS. We need to explore whether there is space here for exemptions from GDPR and for other regulations to be centrally administered, rather than on a piecemeal basis (where each NHS Trust provides individual exemptions for its data). This must, of course, be accompanied by safeguards and oversight of the highest standards, to ensure public confidence, but these safeguarding standards are already being developed in universities and NHS Trusts across the country and will not be a case of reinventing the wheel.
- We should also listen to our citizens’ juries, who have suggested the formation of an independent body of experts to oversee health data sharing within the NHS and with the research community. This would require a collective initiative, led by central government, to build and establish, but would be supported not only by research organisations around the country but by informed public opinion, as evidenced by our juries’ conclusions.
- It is also essential that data collected from electronic health records can be linked with other digital sources. This requires pragmatic adaptations to GDPR and other data protection laws and working with citizens’ juries to understand the public’s response to new forms of data sharing in the health and social care system.
As we look to the future, building and maintaining public confidence in the integrity and effectiveness in the use of patient data must be understood as the first and essential condition for their future development. Without this, we cannot fully benefit from the promise of real-world evidence research and the great advances in research and treatment that they hold.
Corinne Faivre-Finn is Professor of Thoracic Radiation Oncology, The University of Manchester and Honorary Consultant Clinical Oncologist at The Christie. Her interests lie in the development of advanced radiotherapy techniques, combined modality treatments for non small-cell and small-cell lung cancer, real world and electronic patients-reported outcomes.
Niels Peek is Professor of Health Informatics in the Division of Informatics, Imaging and Data Science (School of Health Sciences), and lead for Digital Health and Care at the Christabel Pankhurst Institute for Health Technology Research and Innovation. His research focuses on translational data science for risk prediction, personalised and precision medicine, patient safety, and multimorbidity.
Dr James Price is a PhD Clinical Research Fellow at The University of Manchester and Consultant Clinical Oncologist at The Christie, where he specialises in the treatment of patients with cancers of the head and neck. He is the Clinical Lead for ePROMs roll-out at The Christie.


Saving lives and money through early detection: Lynch syndrome case study
Professor Emma Crosbie

A new national standard for cancer testing in England and Wales (NICE ‘Diagnostic Guidance 42’) was published in October 2020, following a successful policy engagement campaign from my University of Manchester cancer research team and Policy@Manchester. This change means an estimated 1,000 extra people a year with increased risk of cancer can be identified, and then helped with prevention, early detection, and treatment measures. But this is not the end of the story. We need to ensure proper implementation of this policy change, meaning significant changes to testing practices. Healthcare systems here and around the world stand to reap the benefits of the new standard, but we need greater commitment to the expansion and implementation of Lynch syndrome testing.
The importance of testing for Lynch syndrome
Lynch syndrome is a genetic condition that dramatically increases a person’s risk of developing certain cancers, including endometrial (womb) and colorectal (bowel) cancer. The link to bowel cancer was significant enough for the National Institute for Health and Care Excellence (NICE) to establish a national standard (known as ‘Diagnostic Guidance 27’) in 2017, calling for all patients with colorectal cancer to be tested for Lynch syndrome.
Importantly, for every Lynch diagnosis at this stage, it becomes possible to test family members who may also have the condition and are unaware of their increased risk of developing cancer. If they test positive, we can then offer advice on prevention and risk mitigation such as preventive treatments and surgeries and place them into colorectal cancer surveillance programmes. With an aggressive cancer like colorectal cancer, every step to detect and treat earlier is one that will save lives.
My team based in St Mary’s Hospital, Manchester, set out to explore the link between Lynch syndrome and endometrial cancer. We sought to quantify the number of endometrial cancers that are caused by Lynch syndrome and see if a similar national testing standard should be established for all women with endometrial cancer. One distinctive feature of Lynch syndrome is that, for women, endometrial cancer is often the cancer that develops first. This means that, in addition to testing family members for Lynch, we can offer different treatment options and access to colorectal cancer advice and surveillance programmes for women, once we know that their endometrial cancer is Lynch-related.
Findings and recommendations
Our research made four important contributions to understanding the relationship of Lynch syndrome to endometrial cancer:
- We established that the number of endometrial cancers associated with Lynch syndrome was approximately equal to the prevalence of Lynchrelated colorectal cancers. This was central to making the case that endometrial cancers should qualify for the same testing standards;
- We looked at the costs of testing and found that the per-patient cost was well below the financial threshold that our health service defines as affordable;
- We looked at the acceptability of testing – asking women with endometrial cancer if they would want to be automatically offered a Lynch syndrome test, and the overwhelming response was ‘yes’;
- We showed that Lynch syndrome testing led by gynaecological care teams was a feasible method of delivery for healthcare services, with additional psychological benefits to participants.
The next stage for our work was to build the case for policy change. We reached out to Policy@Manchester for their advice on how to get our research findings in front of the right audiences. Our immediate objective was a change to NICE guidance, along the lines of the DG27 recommendation for universal testing of colorectal cancers for Lynch syndrome.
After around 18 months of consultation and evaluation of research evidence, the specialist committee convened by NICE endorsed the principle of universal testing for Lynch syndrome in cases of endometrial cancer.
Turning policy into action
Our success in achieving this new national standard is only the beginning, and with the NICE guidance now in place, our focus turns to implementation. We were encouraged to see new implementation guidance released by NHS England in August 2021. This specifies the diagnostic and testing pathways required to achieve universal testing for Lynch syndrome in cases of both colorectal and endometrial cancer, bringing practice in England and Wales into line with the standards established by NICE guidelines 27 and 42 respectively. Importantly, this guidance also clarifies the commissioning responsibilities (between national and local health systems) for every stage of the pathway. Members of my research team are now participating in a national project to roll out Lynch syndrome testing across the UK.
Our research has shown that, when fully implemented, the move to universal testing for Lynch syndrome in endometrial cancer patients will mean roughly 1,000 new diagnoses in England and Wales every year. Each one of those 1,000 people will have a better chance to prevent and to survive colorectal cancer because they will know about their increased risk. For the families of those whose lives will be saved by joining colorectal cancer surveillance programmes, this represents an incalculable saving. For our health and care systems too, the savings in the long-term from earlier detection and less intensive treatments will easily outweigh the initial investment required to build our testing capacity (infrastructure and personnel) to the level we need.
In terms of future policy, we must quickly move to implement the new NICE guidance here at home and look to share our knowledge and expand these testing standards abroad.
To fully implement NICE guidance:
- We must invest in additional capacity to scale up our existing Lynch syndrome testing sites;
- We need to train enough new pathologists to conduct the testing without diverting resources from the other aspects of their roles;
- We also need to make sure that we can scale up existing colorectal cancer surveillance programmes to accommodate those new men and women whose increased risk is revealed by the test.
Each of these steps will have costs associated with them, but research shows that these will ultimately be paid for by the longer-term savings associated with detecting those cancers more quickly and being able to treat them at an earlier stage.
The challenge of expansion is to find ways to roll out this new standard of testing beyond the UK. While guidelines in the US encourage Lynch syndrome testing in endometrial cancer cases, we have found no similar recommendation in Canada, Australia, or New Zealand. Each of these countries have similarly advanced economies with complex health and care systems, and English as a language of administration. This makes them prime candidates for knowledge transfer based on our existing evidence and policy progress here in the UK. Informing cancer testing standards across Europe is also an important next step. One way in which this could be progressed would be for NICE to bring together a meeting of similar regulatory and advocacy bodies, such as Cancer Council Australia and the Cancer Control Agency (Te Aho o Te Kahu) in New Zealand, to share DG42 and discuss in detail the evidence behind it.
We will continue to push for the adoption of similar guidelines for Lynch syndrome testing in as many healthcare systems as possible, to save lives and costs and showcase the UK’s ability to lead the way in scientific research and cost-effective healthcare policy.
We are already saving lives here at home. With the full implementation of DG42 by cancer testing commissioners, and more investment in testing and surveillance capacity, we can save even more and ultimately do so across the world.
Professor Emma Crosbie is an NIHR Advanced Fellow, Professor and Honorary Consultant Gynaecological Oncologist at The University of Manchester and Manchester University NHS Foundation Trust. She is Cancer Early Detection Lead of the NIHR Manchester Biomedical Research Centre and her research focuses on screening, prevention and early detection of gynaecological cancers.


Advancing cell and gene therapies: Levelling up life sciences investment in the North-West
Professor Fiona Thistlethwaite and Professor Brian Bigger

The UK in general, and Manchester in particular, has been at the forefront of research into advanced cell and gene therapies for cancer and other diseases. These therapies have the potential to revolutionise outcomes for a wide range of diseases with high levels of morbidity and mortality where standard treatments have proved ineffective. The Government seeks to ‘level up’ the north, and one key aspect of that is expanding its growing specialism in life sciences. But are we investing where we need to and in the facilities that we need to in order to turn this ambition into a reality?
Complex decisions for policy-makers
In the health policy world, we are used to talking about QALYs (Quality-Adjusted Life Years), as the standard to decide whether a medication or intervention is acceptable. A QALY means extending a patient’s life by one year of good health, and the National Institute of Health and Care Excellence (NICE) class a treatment worth the money, if it can add QALYs for £20,000–30,000 or less.
Cell and gene therapies upset these calculations in that they are very expensive, but they do offer a potential cure for some cancers. For patients, whose average prognosis was around five more months of life, advanced cell therapies have led to full remission and recovery. As research into these therapies continues to develop, policy-makers will be faced with increasingly complex decisions about how to compare treatments that extend life (via a number of QALYs) and those that could extend life further or even cure a cancer, but at greater immediate expense.
One way that these costs can be reduced is by shortening supply chains and bringing crucial elements of the production processes that underpin these therapies closer to the research and treatment centres that need them. There have been promising investments made in the north by the Department for Business, Energy and Industrial Strategy (BEIS) and the Medical Research Council to advance this agenda. These include the creation of new ‘bioproduction’ and research facilities in Barnsley (cell production), Sheffield (virus production) and the north-east (Darlington – not a production facility but engaged in optimisation work on existing products).
Bioproduction: The role of manufacturing
The way that some advanced cell therapies, such as ‘CAR-T’ and ‘TIL’ immunotherapies, work is that cells are taken from a patient’s body, we replicate those cells (in a bioproduction facility) and they may then be genetically modified using a virus. This virus enables the cell to produce modified proteins that can help the cell to detect and destroy cancer cells or to treat rare genetic diseases. This requires two different manufacturing processes. We need to be able to make the virus, and also to produce the human cells that the virus is inserted into.
There is a distinct lack of this manufacturing capacity in the north of England. We have one facility planned in Sheffield for virus production, and a cell production facility in Barnsley but nowhere nearby for the insertion of the virus into cells, which is its own distinct and specialised process.
Leading the world: 1% of the population, 12% of the trials
Here in Manchester, the Advanced Therapy Treatment Centre (known as iMATCH) has been working to scale up activity in both cell and gene therapy since 2018. Our work at the centre includes looking at the training needs for staff, challenges in setting up advanced cell therapy trials, and how we can translate research insights into treatments for patients. The Advanced Immunotherapy and Cell Therapy team at The Christie currently has twelve open trials, which is the largest number of solid tumour cell therapy trials taking place in a single centre in the UK.
Despite being leaders in pioneering these treatments, in Manchester and across the UK, we face the considerable expense and logistical challenge of having to purchase and often import the cells we need to work with from other countries. With less than one per cent of the world’s population, the UK is currently conducting 12% of global gene therapy trials. This is a distinctive and valuable edge for the UK’s life sciences sector, but one where we are at significant risk of falling behind unless more investment in domestic bioproduction facilities follows.
‘Levelling up’ through life sciences investment?
With the Government’s commitment to ‘levelling up’ the UK’s regions, and the prioritisation of the life sciences sector as part of its wider Industrial Strategy, now is the time to push for investment in new bioproduction facilities in the north of England. We welcome the investment that led to the virus production facility in Sheffield, but viruses are only one of the two necessary elements for advanced cell therapies. Cell therapy requires both virus production and production capacity for the cells that deliver the virus into the body, making a cell production facility in the north the logical next step to build on that investment.
There are a number of reasons that Greater Manchester in particular stands out as a strong candidate for the location of such a facility:
- A large population of students with experience in biomedical disciplines. This is a valuable skills base that can form a natural talent pipeline for the specialist jobs that a bioproduction plant would create;
- Greater Manchester is well placed for transport, with established links to London and across the Northern Powerhouse region via the ‘M62 Corridor’;
- The University of Manchester is home to the central hub of the national Royce Institute for Advanced Materials. Materials being produced by the Royce, such as hollow-fibre materials for advanced filtration, are already bringing down market prices for the advanced materials needed for this work, and their proximity will allow for greater collaboration and development of new materials in future;
- Manchester is an advanced therapy training centre with several MSc and PhD level programmes in the biotechnology sector, including iMATCH, and already offers specialised hands-on training for the specialised workforce required.
Regardless of its exact location, a cell production facility in the north would create highly skilled jobs, enhance our national research capacity, shorten existing supply chains, and add to the existing life sciences industrial specialisation across Cheshire, Greater Manchester and beyond.
The freedom to set our own regulatory environment for life sciences research in the UK since Brexit is a key strand of government thinking on the future of this sector in the UK economy. To attract more international investment in drug development and human trials, we need to build our own cell production infrastructure, rather than relying on importing the materials we need from the US and other countries.
The US provides one potential model for the UK government to follow. In California, the California Stem Cell Initiative at UCLA was pump-primed with significant federal investment. What followed was the organic development of a life sciences industry cluster, with pharmaceutical companies and research organisations opening laboratories and offices close by. With the Government commitment and industry partnerships underpinned by the post-Brexit Life Sciences Sector Deal, the UCLA example could be replicated here in northern England.
The Office for Life Sciences (a government body ran jointly between BEIS and the Department for Health and Social Care) is best placed to catalyse government engagement with the strategic economic and health benefits that a new bioproduction facility will deliver. We encourage the Office for Life Sciences to convene a working group with representatives from government, industry, research and clinical practice to examine the California/UCLA precedent and its potential application to a new bioproduction facility in the north-west.
With leadership from both national and cityregional government, the next expansion of the northwest’s significant life sciences sector will underwrite new breakthroughs in advanced research, new treatments for many deadly cancers, new high-skill/high-wage jobs, and secure UK leadership in international life sciences investment and exports. The benefits are simply too great for this decision to be delayed or postponed any longer.
Fiona Thistlethwaite is a Medical Oncology Consultant within the Experimental Cancer Medicine Team (ECMT), Advanced Immunotherapy and Cell Therapy (AICT) Team Clinical Lead, Honorary Professor at The University of Manchester, and Director of iMATCH (Innovate Manchester Advanced Therapy Centre Hub). She has an active clinical trials research portfolio in early phase clinical trials (adult solid tumours) within the ECMT and AICT
Brian Bigger is Professor of Cell and Gene Therapy in the Division of Cell Matrix Biology and Regenerative Medicine at The University of Manchester. His Stem Cell and Neurotherapies lab works on the development and delivery of clinical cell and gene therapies for rare genetic diseases and brain tumours. He is also Chairman of the European Study Group on Lysosomal Diseases (ESGLD).


Global partnerships: How can international research collaborations boost our health and economy?
Professor Keith Brennan, Dr F. George Njoroge, and Professor Robert Bristow

International collaborations are one of the cornerstones in cancer research. They enable us to improve how we meet major health challenges through developing reciprocal relationships and sharing learnings with others around the world. Research that crosses international borders is a fundamental building block of ‘precision medicine for all’ – tailoring medicines and treatments to work best according to an individual’s genetic makeup. The wider our research can reach, the greater the number of patients, at home and abroad, who will ultimately benefit. So, what are the barriers to successful international collaborations and what can we do to improve outcomes and take advantage of the opportunities?
Cuts to aid and addressing ethnic inequality
The cut in the UK’s Official Development Assistance (ODA) funding (often referred to as the overseas aid budget), from 0.7 to 0.5% of our Gross National Product, has increased barriers to funding for UK based international research projects. While research investment (for example, the Global Challenges Research Fund) is only one pot of international aid funded through ODA, it has been disproportionately cut back. UK Research and Innovation announced in March 2021 that the reduction meant a £120 million shortfall for projects that they had already committed to supporting. This means that many approved research projects are facing budgets cut or cancellation, and that the amount of support for future proposals has been substantially reduced. The recent announcement that the 0.7% commitment will be restored in Financial Year 2024/25 will do little to address the present impacts and ongoing consequences of the cut, which will be felt across UK international scientific research for years to come.
These funds have historically played a key role in addressing the gap in knowledge about how to treat cancers across different ethnicities. Currently most UK-based cancer research focuses mainly on people of European descent because this accounts for most participants in clinical trials. This leaves the genetic variabilities of different heritages under-explored. The implications are far reaching, for example, certain immunotherapies might be proved effective and safe for patients of European descent, but the same doses might be toxic in African populations. We need to tailor treatments for different populations and use genetic analysis to identify how tumours respond to different methods of treatment.
Funding research that looks at populations with a variety of genetic descent is therefore essential to improved outcomes, not just for other countries but to also better serve our population here in the UK. Progressing this research will move our treatment towards precision oncology for all patients, meaning that patient outcomes should improve significantly and a major health inequality in cancer services will begin to be addressed.
Barriers to research partnerships: practical considerations
One barrier to multinational collaborations is the lack of an agreed set of minimum ethical standards, as the research techniques developed in one country might not be acceptable in another. Ethics approval processes, required as part of any major research project (particularly ones involving health and testing/ treating people), take more time than should be the case. The ease with which research ideas and data can be transferred between international partners can also pose an obstacle to research progression. Clear standards relating to the holding, organising and sharing of data and a consistent mechanism for data transfer between countries would allow more proactive collaborations.
Another barrier is healthcare recruitment. The UK currently benefits from recruitment of nurses from overseas, for example from Kenya. Taking trained healthcare professionals to fill shortfalls here saves the UK around £50,000 to £60,000 per person in training costs. However, this relationship depletes the healthcare capacity of their home countries and we need to find ways to give back to these countries in a way that equally benefits them. We could, for example, be upskilling clinical services overseas through education, training and capacity building.
By upgrading cancer services overseas, our economy can also benefit through becoming a provider of training, medicines and equipment like mammography machines. Building diagnostic and treatment capacities in other countries through international partnerships is, at the same time, building a future market for life sciences exports and health technologies developed here. Also, if workforces overseas are scaled up and improved, UK-based companies have more opportunities for placing sites in these areas and scaling up production.
Leading by example: a collaboration with Kenya
Here in Manchester, there are numerous international collaborations committed to improving cancer outcomes here in the UK and internationally. The Manchester Cancer Research Centre’s partnership with The Kenya UK Healthcare Alliance and the Kenyatta University Teaching, Referral and Research Hospital (KUTRRH) is working to improve clinical services for cancer in Kenya by using the ‘hub and spoke’ model developed by The Christie and local cancer services across Greater Manchester. This model enables a centre of excellence (the hospital), to support treatment in a number of other healthcare settings, ensuring the same high treatment standards for individuals across a large geographical area, no matter where they are treated.
Another area of international collaboration focuses on work to understand genetic factors in certain cancers. In Kenya, researchers affiliated to our international collaboration collected samples from prostate cancers in order to sequence Kenyan cancer genomes and understand genetic and environmental drivers of the disease in the country. As a result, we have been able to improve treatment pathways for prostate cancers for patients by taking the genetic differences of Kenyan cancers into account. This is a clear example of our move towards precision oncology for all.
Policy leadership for international success
The practical issues facing international collaborations require national leadership to cut through the complexities of the issues. Steps that could be taken include:
- New pre-approval ethics arrangements. For example, the Manchester Cancer Research Centre negotiated an ‘umbrella’ ethics agreement on the use of patient data with Manchester University NHS Foundation Trust. This granted a provisional pre-approval, based on the research organisation satisfying certain conditions about use and safeguarding of data. UK Research and Innovation is a national body well-placed to lead a work programme exploring the possibility of similar pre-approval ethics arrangements for international collaborations – subject to an agreed set of minimum ethics and data standards.
- Reconsideration around ODA funding. The current reduction in ODA funding has already negatively affected our ability to create and conduct worldleading international research. The Government’s commitment to returning to 0.7% of Gross National Product is welcome, but will not mitigate the effects of this cut to current research activities. Recalibrating the existing budget, with an immediate compensatory uplift in the research component going forward, is a reasonable and practical step towards safeguarding the valuable work that we have already been able to do.
- Establish greater equity and equality in reciprocal healthcare arrangements. The Department of Health and Social Care and Health Education England should work together to ensure that the international recruitment of healthcare workers is partnered with reciprocal arrangements for remote training and upskilling for staff in their home countries. A programme could be designed, at minimal additional cost to the taxpayer, that adds a new international equity to an arrangement that benefits our NHS, often to the cost of domestic health services.
- Be ambitious and explore the opportunities. We must not neglect the mutual benefit and economic growth that international research collaborations can bring to both countries involved. We can show how research funds translate not only into better outcomes at home and abroad, but greater economic opportunities. With leadership from the Department for Business, Energy and Industrial Strategy (focusing on our life sciences sector here in the UK) and the Department for International Trade (looking at export and development opportunities across the world), we could build a consensus between research, industry and policy-makers to turn recommendations into reality.
With greater investment and fewer barriers to international research, the UK could develop new industrial bases and export relationships for our businesses, at the same time as learning more about how to diagnose and treat diseases like cancer. There is no time to waste. The full potential of international research collaborations should be realised.
Keith Brennan is the Associate Dean for Internationalisation in the Faculty of Biology, Medicine & Health and Professor of Developmental Signalling in the Division of Cancer Sciences at The University of Manchester. He is particularly interested in how developmental signalling pathway, which control cellular behaviours like proliferation, apoptosis, adhesion and fate, are disrupted during tumour initiation and breast cancer progression.
Dr F. George Njoroge is a member of Board of Directors and Chairman of Scientific Research and Innovation Committee at Kenya Medical Research Institute (KEMRI). He was a former Chief Scientific Adviser at Kenyatta University Teaching, Referral & Research Hospital (KUTRRH). Previously, a Senior Research Fellow at Eli Lilly and Company and a former Director in the Department of Medicinal Chemistry at Merck Research Laboratories. George is an avid drug hunter with a wealth of drug discovery in infectious and cancer therapeutic areas.
Robert Bristow is Director of the Manchester Cancer Research Centre, Professor of Cancer Studies in the Division of Cancer Sciences (The University of Manchester) and Chief Academic Officer at The Christie. His Translational Oncogenomics laboratory within the CRUK Manchester Institute focuses on the role of the tumour microenvironment on therapeutic resistance and metastases and the genetic basis for aggression in both sporadic and hereditary prostate cancers.

With special thanks to our contributors:
Dr Philip Crosbie is a Senior Lecturer in the Division of Infection, Immunity and Respiratory Medicine at The University of Manchester and an Honorary Consultant in Respiratory Medicine based at Wythenshawe Hospital, Manchester University NHS Foundation Trust. His research and clinical focus is the early detection of lung cancer.
Dr Suzanne Johnson is a lecturer in the Division of Cancer Sciences and Division lead for Social Responsibility with particular interests in developing inclusive practice in research and teaching and addressing health inequities. Her background is in basic and translational cancer research, and in 2020 Suzanne was appointed Programme Director to develop a new online Masters programme in Transformative Oncology with The University of Manchester Worldwide.
Professor David Shackley is the Clinical Lead at MAHSC (Manchester Academic Health Science Centre) for cancer working with colleagues to bring research and clinical care closer together. He is the Director of the Greater Manchester Cancer Alliance, and a Consultant Urological Surgeon. From 2015 to 2018 he was the Medical co-lead for the National Cancer Vanguard which brought GM and London together as a 10 million catchment population to develop and test new cancer approaches before a broader roll out across NHS England.
Dr Dónal Landers is Director of the digital Experimental Cancer Medicine Team, Cancer Research UK Manchester Institute. He is a Clinician and a Fellow of the Faculty of Pharmaceutical Medicine with over 25 years of experience and achievement in early clinical development, clinical practice, healthcare and pharma consulting, and delivering digital health innovation and solutions.
Karen Kirkby is Professor of Proton Therapy Physics - a joint post between The University of Manchester and The Christie. Karen is responsible for developing a programme of international leading proton research and innovation to deliver direct patient benefits. This goes from basic research, through pre-clinical and translational research to clinical trials.
Professor Ananya Choudhury is Chair and Honorary Consultant in Clinical Oncology at The Christie and The University of Manchester. Ananya specialises in urology and has a strong interest in translational research. She is clinical lead for advanced radiotherapy including the MR-LINAC project and is co-Group Leader of The Translational Radiobiology Group within the Division of Cancer Sciences.
Dr Gareth Price is a Senior Lecturer in the Division of Cancer Sciences at The University of Manchester and a clinical scientist at The Christie. His research focuses on the use of data collected during routine care to derive clinical insight, identify potential improvements in treatment, and provide evidence of the impact of innovations and changes to practice on patients’ clinical outcomes.
Corinne Faivre-Finn is Professor of Thoracic Radiation Oncology, The University of Manchester and Honorary Consultant Clinical Oncologist at The Christie. Her interests lie in the development of advanced radiotherapy techniques, combined modality treatments for non small-cell and small cell lung cancer, real word and electronic patients-reported outcomes.
Niels Peek is Professor of Health Informatics in the Division of Informatics, Imaging and Data Science (School of Health Sciences), and lead for Digital Health and Care at the Christabel Pankhurst Institute for Health Technology Research and Innovation. His research focuses on translational data science for risk prediction, personalised and precision medicine, patient safety, and multimorbidity.
Dr James Price is a PhD Clinical Research Fellow at The University of Manchester and Consultant Clinical Oncologist at The Christie, where he specialises in the treatment of patients with cancers of the head and neck. He is the Clinical Lead for ePROMs roll-out at The Christie.
Professor Emma Crosbie is an NIHR Advanced Fellow, Professor and Honorary Consultant Gynaecological Oncologist at The University of Manchester and Manchester University NHS Foundation Trust. She is Cancer Early Detection Lead of the NIHR Manchester Biomedical Research Centre and her research focuses on screening, prevention and early detection of gynaecological cancers.
Fiona Thistlethwaite is a Medical Oncology Consultant within the Experimental Cancer Medicine Team (ECMT), Advanced Immunotherapy and Cell Therapy (AICT) Team Clinical Lead, Honorary Professor at The University of Manchester, and Director of iMATCH (Innovate Manchester Advanced Therapy Centre Hub). She has an active clinical trials research portfolio in early phase clinical trials (adult solid tumours) within the ECMT and AICT.
Brian Bigger is Professor of Cell and Gene Therapy in the Division of Cell Matrix Biology and Regenerative Medicine at The University of Manchester. His Stem Cell and Neurotherapies lab works on the development and delivery of clinical cell and gene therapies for rare genetic diseases and brain tumours. He is also Chairman of the European Study Group on Lysosomal Diseases (ESGLD).
Keith Brennan is the Associate Dean for Internationalisation in the Faculty of Biology, Medicine & Health and Professor of Developmental Signalling in the Division of Cancer Sciences at The University of Manchester. He is particularly interested in how developmental signalling pathway, which control cellular behaviours like proliferation, apoptosis, adhesion and fate, are disrupted during tumour initiation and breast cancer progression.
Dr F. George Njoroge is a member of Board of Directors and Chairman of Scientific Research and Innovation Committee at Kenya Medical Research Institute (KEMRI). He was a former Chief Scientific Adviser at Kenyatta University Teaching, Referral & Research Hospital (KUTRRH). Previously, a Senior Research Fellow at Eli Lilly and Company and a former Director in the Department of Medicinal Chemistry at Merck Research Laboratories. George is an avid drug hunter with a wealth of drug discovery in infectious and cancer therapeutic areas.
Robert Bristow is Director of the Manchester Cancer Research Centre, Professor of Cancer Studies in the Division of Cancer Sciences (The University of Manchester) and Chief Academic Officer at The Christie. His Translational Oncogenomics laboratory within the CRUK Manchester Institute focuses on the role of the tumour microenvironment on therapeutic resistance and metastases and the genetic basis for aggression in both sporadic and hereditary prostate cancers.
Analysis and ideas on cancer, curated by Policy@Manchester.
Read more and join the debate at: blog.policy.manchester.ac.uk www.policy.manchester.ac.uk
For more information about our cancer research, go to: https://www.manchester.ac.uk/cancer-beacon
The opinions and views expressed in this publication are those of the respective authors and do not necessarily reflect the views of The University of Manchester.
All opinions and recommendations made by article authors are made on the basis of their research evidence and experience in their fields.
Evidence and further discussion can be obtained by correspondence with the authors; please contact policy@manchester.ac.uk in the first instance.